What Determines an Element's Emission Spectrum Apex
Ive heard that it may have something to do with the electron energy levels but I dont know if that is correct. These emission spectra are as distinctive to each element as fingerprints are to people.

Photoluminescence Emission Spectrum For Aln Samples With Varying Carbon Download Scientific Diagram
You can look at the spectra and identify which elements are present.

. Each transition has a specific energy difference. The emission spectrum shows the different colors of light that an element emits. The electrons gained energy from the heat of a flame to go to a higher energy level then fall back to their original lower energy level.
The emission spectrum shows the different colors of light that an element emits. The characteristic spectrum of colors emitted by an atom. The emitted light corresponds to energies of the specific electrons.
More differences between absorption and emission spectrum are given below in a tabular column. Calculating the wavelength of an energy transition. Each element has its own unique atomic emission spectrum.
A gas of hydrogen atoms will produce an absorption line spectrum if it is between you your telescopespectrograph and a continuum light source and an emission line spectrum if viewed from a different angle. The EM wave with the shortest wavelength and the most dangerous EM wave. This collection of transitions makes up an emission spectrum.
Analyzing the spectrum of a star you can figure out what elements are present and also get an estimate on how. A given atom will absorb and emit the SAME frequencies of electromagnetic E-M radiation. I know that every element has a different emission spectra but what determines the wavelengths present in a spectra of any given element.
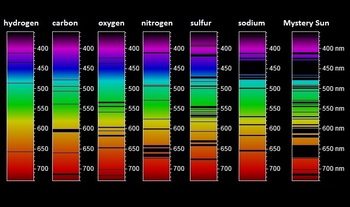
The line at 5890 has twice the intensity of the line at 5896 nm. The emission spectrum of each element has characteristic lines for each element. The width of the line can tell us how fast the material is moving.
The main difference between emission and absorption spectra is that an emission spectrum has different coloured lines in the spectrum whereas an absorption spectrum has dark-coloured lines in the spectrum. From the energy level diagram it can be seen that these lines are emitted in a transition from the 3p to the 3s levels. N2 n1 1.
Id like to know what causes the wavelengths of the emission spectra of different elements. The photon energy of the emitted photon is equal to the energy difference between the two states. If you were to observe the star a source of white light directly you.
Therefore when you are measuring the emission spectrum of an element only certain wavelengths of light are allowed and the pattern that is produced is unique for that substance. Is used to study the interaction of matter and energy. Atoms molecules or ions that absorb radiation are known to be in an excited state.
Test your knowledge on Emission spectrum. ν 109677 1 n 1 2 1 n 2 2 Where n1 1234. There are many possible electron transitions for each atom.
The number of times a wave vibrates or passes a fixed point per second. The emission spectrum of a chemical element or chemical compound is the spectrum of frequencies of electromagnetic radiation emitted due to an electron making a transition from a high energy state to a lower energy state. View Homework Help - Quiz.
ν wavenumber of the electromagnetic radiation. Atomic emission spectra are produced when excited electrons return to ground state. The value 109677 cm -1 is known as Rydberg constant for hydrogen.
The emission lines correspond to the differences between various pairs of the many energy levels. Each elements emission spectrum is distinct because each element has a different set of electron energy levels. Every element in the periodic table has a distinct set of electron energy levels so for a given element only photons of specific energies can be emitted.
Other hydrogen atoms can have the electron excited into the n 4 shell. Spectra from CHEMISTRY Honors Che at Apex High. When the electrons fall back to their resting states they emit photons of light.
We can learn about winds in stars from this. The lines photons are emitted as electrons fall from higher energy orbitals to lower energies. Then they jump back down again.
Emission Spectra VS Absorption Spectra. Different elements produce different line spectra. The general formula for the hydrogen emission spectrum is given by.
A line spectrum is like a fingerprint - it can be used to identify the element present. Different elements emit different emission spectra when they are excited because each type of element has a unique energy shell or energy level system. Certain compounds and elements burn with distinctive colors.
The number of protons B. When an atom absorbs energy its electrons jump to higher energy levels. Spectra Question 10 2 points What determines an elements emission spectrum.
The emission spectra of an element is the spectrum of radiation emitted due to an atom or molecule absorbing energies and transitioning from a high energy state to a lower energy state. Sodium Atomic Emission Spectrum The sodium spectrum is dominated by the bright doublet known as the Sodium D-lines at 5889950 and 5895924 nanometers. There are many possible electron transitions for.
The electron in some hydrogen atoms may be excited into the n 2 level. From spectral lines astronomers can determine not only the element but the temperature and density of that element in the star. Atomic emission spectrum led development the atomic theoryFAQexplain how the understanding atomic emission spectrum led development the atomic theoryadminSend emailDecember 13 2021 minutes read You are watching explain how the understanding.
What is an emission spectrum apex. Each jump corresponds to a particular wavelength of light. How Elements Produce Emission Spectra When elements are vapourised and heated their electrons jump in energy level.
The emission spectrum of a star is the spectrum of frequencies for emitted electromagnetic radiation during the transition of an atoms electrons from a high-energy state to a low-energy state. The spectral line also can tell us about any magnetic field of the star. Line emission spectra are unique to a particular element.

Pin By Danielle Profenno On Science What Not Chemistry Classroom Chemistry Lessons Science Chemistry
What Is An Emission Spectrum Example

Pdf A Review Of Main Characterization Methods For Identifying Two Dimensional Organic Inorganic Halide Perovskites

A Diffuse Reflectance Transmittance Spectra Of Yag X Ce 3 X 0 2 Download Scientific Diagram
Why Is Each Element S Emission Spectrum Unique Quora

Trlfs Data Deconvolution 10 5 M Eucl 3 And 7 10 8 To 7 10 5 M Cam Download Scientific Diagram
How Does Quantum Mechanics Explain The Emission Spectra Of Atoms And Molecules Quora
What Is An Emission Spectrum Example

Elemental Emission Lines Used In The Spectral Fingerprinting Of The Oysters Download Table
Why Is Each Element S Emission Spectrum Unique Quora

What Determines An Element S Emission Spectrum O A The Size Of The Sample O B The Mass Of The Brainly Com
Solved Use The Emission Spectra Shown To Answer The Following Questions Note The Direction Of The Wavelength Scale Screenshot With The Emission Course Hero

Pdf Libs A Geochemical Tool For The 21st Century
What Is An Emission Spectrum Example
Why Is Each Element S Emission Spectrum Unique Quora

Advancing Understanding Of Actinide Iii Ac Am Cm Aqueous Complexation Chemistry Chemical Science Rsc Publishing Doi 10 1039 D1sc00233c

Solid State Synthesis Of Ph4p Mi3 M Eu2 Sr2 Sn2 And Investigation Of Photoluminescence Properties Of Green Emitting Phosphor Siai 2021 European Journal Of Inorganic Chemistry Wiley Online Library
Comments
Post a Comment